The ultralow temperature facility in Bayreuth
consists of four nuclear magnetic refrigerators for microkelvin temperatures and four
3He-4He dilution refrigerators for millikelvin temperatures.
The laboratory has achieved the lowest equilibrium temperature available for condensed
matter physics experiments, its refrigerators have the largest cooling capacity for
ultralow temperatures and has the largest number and variety of refrigerators for micro-
and millikelvin temperatures.
The inhouse experience for users experiments consists of:
Nuclear magnetic refrigeration, dilution refrigeration, thermometry at ultralow
temperatures, vacuum technology, electronics for ultralow temperatures, nuclear magnetic
resonance, acoustic technologies, calorimetric experiments, SQUID technology,
magnetometry, thermal contact and conductivity, electrical impedance, mechanical
properties of solids.
Temperature is a physical quantity which gives us an idea of how hot or
cold an object is. The temperature of an object depends on how fast the atoms and
molecules which make up the object can shake, or oscillate. As an object is cooled, the
oscillations of its atoms and molecules slow down. For example, as water cools, the
slowing oscillations of the molecules allow the water to freeze into ice. In all
materials, a point is eventually reached at which all oscillations are the slowest they
can possibly be. The temperature which corresponds to this point is called absolute zero.
Note that the oscillations never come to a complete stop, even at absolute zero.
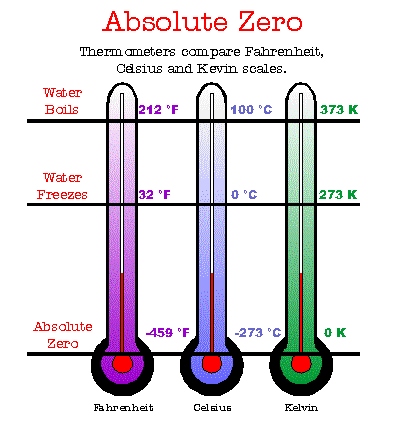
There are three temperature scales. Most people are familiar with either the Fahrenheit or
the Celsius scales, with temperatures measured in degrees Fahrenheit (� F) or degrees
Celsius (� C) respectively. On the Fahrenheit scale, water freezes at a temperature of
32� Fahrenheit and boils at 212� f. Absolute zero on this scale is not at 0�
Fahrenheit, but rather at -459� Fahrenheit. The Celsius scale sets the freezing point of
water at 0� Celsius and the boiling point at 100� Celsius. On the Celsius scale,
absolute zero corresponds to a temperature of -273� Celsius.
Scientists - especially those who study what happens to things when they become very, very
cold - commonly use the Kelvin scale, with temperatures measured in Kelvin (K). This scale
uses the same temperature steps as the Celsius scale, but is shifted downward. On this
scale, water freezes at 273 K and boils at 373 K. Only on the Kelvin temperature scale
does absolute zero actually fall at 0 K.